
What we stand for
Quantoom Biosciences is a full-stack RNA partner for mRNA- and saRNA-based vaccines and therapeutics. Its N-Force toolbox relies on 3 core elements to turn any antigen into a (sa)mRNA-LNP drug product: Ncode for sequence design and optimization, Ntensify® for RNA production and Ncapsulate® for RNA-LNP formulation. Beyond technology, Quantoom Biosciences assists its partners by providing extensive enabling solutions, ranging from strategic R&D partnerships to sequence design & optimization.
Our expertise & offering
Our world-class team of 40+ people bring together a depth and breadth of knowledge born from years of practical experience in the seamless scale-up of technology for bioprocessing manufacturing. Drawing from a collective decade-long track record in biomanufacturing, acquired at our parent company Univercells. Our team’s diverse skill set has equipped them to handle a diverse range of challenges and deliver innovative solutions.

Expertise in RNA
To support our mission of removing the barriers to making accessible and affordable mRNA- and saRNA-based vaccines, our international team is composed of experts with competencies ranging from in-silico to in-vivo experimental studies and analytical tools. Our brilliant minds have strong and complementary backgrounds in molecular and structural biology, process development, equipment engineering, qualification and validation, product development, and more.
Expertise in
Bioprocessing
Based on fundamental engineering principles, the idea is to intensify and introduce sequential-staggered production. This significantly reduces the footprint for easy transportation and optimization of cleanroom space. Manual steps are reduced which improves safety and product quality by advancing RNA production technologies, Quantoom Biosciences will drastically reduce production costs and improve the availability of life-saving biologics.


Expertise in
Formulation
Quantoom has built a highly-skilled team of international experts to develop Ncapsulate™, our RNA formulation system. The team can manage the development, screening and optimization of lipid nanoparticle (LNP) and polymer-based formulations for RNA delivery. We can also provide physicochemical, in vitro and in vivo characterization, including biodistribution and immune challenge for prophylactic vaccine application of RNA delivery systems. We provide services for the development of the entire RNA formulation process and manufacturing system.
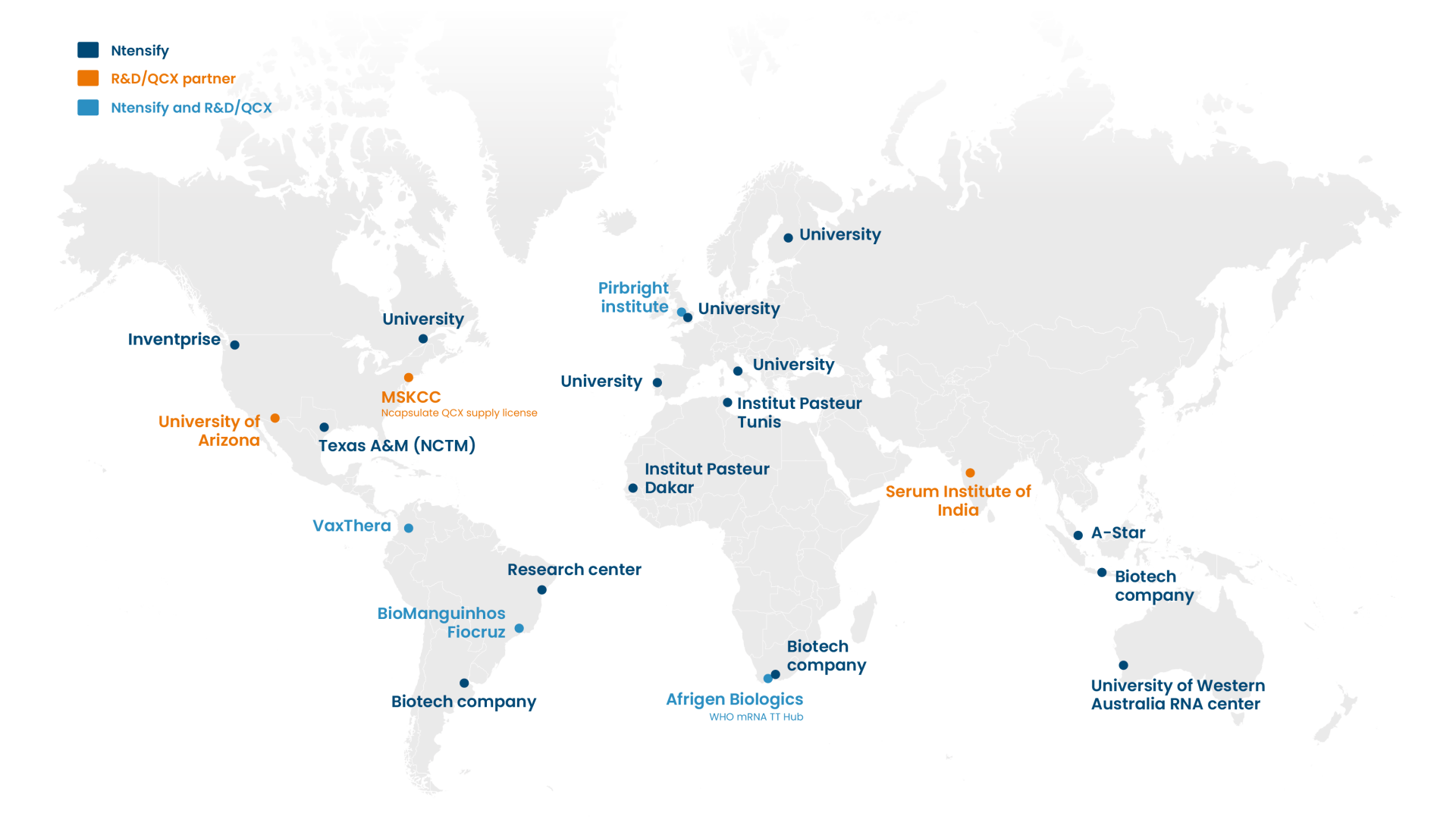
Our partners
Our global collaborations
Since its commercial launch in 2023, Quantoom Biosciences has installed 25 Ntensify® systems worldwide and has five ongoing R&D collaborations.
Diversity & values
At Quantoom Biosciences, we stand for inclusivity, diversity, and respect for individual differences. We strive to practice equality across the board, from our hiring methods to how we pay employees. Our team represents many diverse backgrounds and perspectives.
Accountability
We own and assume accountability for results, we establish high-performance standards
Continuous improvement
We challenge the status quo, embrace innovation, add value, and deliver operational excellence
Teach/lead
We value and solicit new ideas, develop and refine competencies by sharing knowledge and experience
Integrity
We maintain high ethical standards; respect, include and recognize differences
Team
Everyone acts as a team builder, we remove the barriers that block the team’s success
Our team
Management Team
Click on each picture to see the bio of each member

Jose Castillo, PhD.
Chief Executive Officer

Rachana Talwar
Chief of staff & Chief Operating Officer

Ashiqul Haque, PhD.
Chief Scientific Officer

Coralie Pirlot
Chief Financial Officer

Hala Audi
Global Director Alliance & Partnership

Noémie Jacquart
Head of Marketing & Sales Operations

Youlia Serikova, PhD.
Head of Process Development

Laurent Heusgens
Head of Engineering and Services

Maria Davila-Saignol
Global Director Sales & Business Dev.
Scientists
Our team of scientists brings expertise across a broad spectrum of capabilities
Our history
Quantoom Biosciences is a full-stack RNA partner for mRNA- and saRNA-based vaccines and therapeutics. Built around our N-Force toolbox — Ncode for sequence design, Ntensify® for scalable RNA production, and Ncapsulate® for RNA-LNP formulation — our platform turns any antigen into a high-quality (sa)mRNA-LNP drug product. Beyond technology, Quantoom Biosciences supports partners with extensive enabling services, from strategic R&D collaborations to sequence design and optimization.
University of Western Australia selects Ntensify® mini for advanced RNA research
The University of Western Australia’s RNA Innovation Foundry selects Quantoom Biosciences’ Ntensify® mini platform to support cutting-edge RNA research and technology development. This adoption highlights Quantoom’s growing footprint in academic research and innovation ecosystems, and its role in enabling the next generation of RNA-based therapies.
Restructuring and creation of Phoenix Biosciences as a standalone company
Quantoom Biosciences enters a new phase under the legal entity Phoenix Biosciences SA, through which all activities and technologies now continue. This restructuring provides a clear and solid foundation for the future, strengthening our position as a full-stack RNA solutions provider.
Research collaboration with Leading Global Cancer Center
Quantoom Biosciences signs a research license and supply agreement with Memorial Sloan Kettering Cancer Center to support the development of personalized mRNA-based cancer therapeutics. This collaboration confirms Quantoom’s expansion into high-value clinical research and oncology applications, reinforcing the versatility of its mRNA formulation and manufacturing technologies.
Launch of the Ntensify micro
Quantoom Biosciences launches the Ntensify® micro, expanding the R&D segment of its Ntensify® product line. The Ntensify® micro is the smallest and most compact system designed for the Ntensify® process. This benchtop unit enables the production and purification of 500 µg to 100 mg of high-quality mRNA in just 4 to 6 hours.
Belgium and Brazil unite to advance RNA-based therapies
Quantoom Biosciences signs a Memorandum of Understanding with Bio-Manguinhos/Fiocruz, strengthening international cooperation to accelerate the development and local production of RNA-based therapeutics. This milestone reflects Quantoom’s growing role as a global technology enabler, supporting sovereign manufacturing strategies beyond vaccines.
Strategic collaboration with Serum Institute of India
Quantoom Biosciences enters into a strategic collaboration with the world’s largest vaccine manufacturer to expand the accessibility of RNA-based personalized oncology and immunotherapies. This partnership marks a major step beyond vaccines, demonstrating Quantoom’s ability to support point-of-care, small-scale mRNA manufacturing for advanced therapeutic applications worldwide.
Launch of the Ntensify mano
Quantoom Biosciences announces the extension of the Ntensify product line with the launch of Ntensify mano, an mRNA kit containing mixes of reagents for mRNA production and purification for research use.
Installation of the 1st Ntensify midi
Quantoom Biosciences announces the installation of its first Ntensify midi system for mRNA vaccine production in the GMP facility of Afrigen Biologics in Cape Town, South Africa. (under 19 months of dev…)
Agreement for the first African-owned Covid-19 vaccine
The collaboration with Afrigen Biologics (hosting the World Health Organization’s Global mRNA Vaccine Technology Transfer Hub) will focus on the development of a novel mRNA vaccine that will drastically accelerate access to vaccination by using Quantoom’s mRNA production technology.
Collaboration with eTheRNA
Quantoom entered into a strategic collaboration to build an advanced, small footprint technology platform for the affordable production of RNA-based therapies. This platform was designed to be used either within existing facilities or rapidly deployed to areas of urgent need.
Official launch of Quantoom Biosciences
The COVID-19 pandemic underlined the desperate need for technological innovation in manufacturing to provide a simple, scalable way to make RNA. By lowering the barriers to RNA production, we allow our partners that have previously not considered exploring RNA-based vaccines and therapeutics, to gain access to RNA production capabilities and enter the market.






